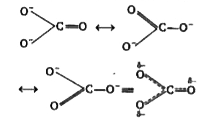
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The incorrect statements among the following is I. NCl(5) does no...
Text Solution
|
- The incorrect statements among the following is/are (I) NCl5 does not ...
Text Solution
|
- Why does phosphorus exist as PCl(5) but nitrogen cannot exist as NCl(5...
Text Solution
|
- Why PCl(5) exists but NCl(5) does not ?
Text Solution
|
- PCl(5) is known but NCl(5) is not known. Or Nitrogen does not form p...
Text Solution
|
- PCl(5) एक ज्ञात यौगिक है जबकि NCl(5) ज्ञात नहीं है ।
Text Solution
|
- NCl(3) संभव है लेकिन NCl(5) संभव नहीं है जबकि PCl(3) व PCl(5) दोनों सं...
Text Solution
|
- In the incorrect statement/s among the following is/are I. NCl(5) do...
Text Solution
|
- PCl(5) exists but NCl(5) does not due to
Text Solution
|