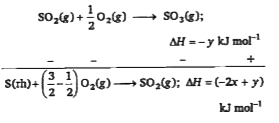A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Using the following thermocchemical equations (i) S(g)+3//2O(2)(g)...
Text Solution
|
- The reaction SO(2)(g) +1//2O(2)(g) rarr SO(3)(g) should be
Text Solution
|
- Given below are the values of DeltaH^(ɵ) and DeltaS^(ɵ) for the reacti...
Text Solution
|
- If C(s)+O(2)(g)rarrCO(2)(g),DeltaH=X and CO(g)+1//2O(2)(g)rarrCO(2)(g)...
Text Solution
|
- निम्नलिखित ऑकड़ों के आधार पर कार्बन मोनोक्साइड (CO) के गठन की ऊष्मा (य...
Text Solution
|
- If C(s)+O(2)(g)to CO(2)(g),DeltaH=rand CO(g)+1/2O(2)to CO(2)(g),DeltaH...
Text Solution
|
- निम्नलिखित ऊष्मा-रासायनिक अभिक्रियाओं से H(2)Sकी संभवन ऊष्मा का मान ज्...
Text Solution
|
- Three thermochemical eq^(ns) are given below 1)C("graphite")+O(2(g)...
Text Solution
|
- Given (at 25^(@)C ): 2FeSO(4)(s)toFe(2)O(3)(s)+SO(2)(g)+SO(3)(g),Delta...
Text Solution
|