Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
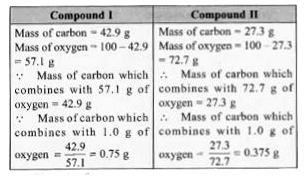
- Carbon and oxygen are known to form two compounds. The carbon content ...
Text Solution
|
- Carbon and oxygen are known to form two compounds. The carbon content ...
Text Solution
|
- Hydrogen and oxygen are known to from two compounds. The hydrogen cont...
Text Solution
|
- Sulphur and oxygen are known to form two compounds. The sulphur conten...
Text Solution
|
- Carbon and oxygen forms two compound . Carbon content in one of them i...
Text Solution
|
- Carbon and oxygen form two compounds. Carbon content in one of them is...
Text Solution
|
- Carbon is found to form two oxides, which contain 42.9% and 27.3% if c...
Text Solution
|
- In two compound of hydrogen and oxygen, hydrogen present in 42.9% and ...
Text Solution
|
- Hydrogen combines with oxygen and forms two compounds. In the first co...
Text Solution
|