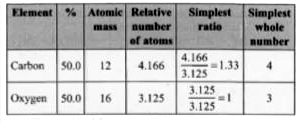
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A Carbon compound containing only carbon and oxygen has an app...
Text Solution
|
- An organic compound contains 14 atoms of carbon per molecule. If mass ...
Text Solution
|
- An organic compound contains 14 atoms of carbon per molecules. If mass...
Text Solution
|
- A compound containing only carbon, hydrogen and oxygen has molecular m...
Text Solution
|
- A carbon compound containing only carbon and oxygen has an approximate...
Text Solution
|
- एक यौगिक केवल C तथा O से बना है । विश्लेषण करने पर यह पाया गया कि दोनो...
Text Solution
|
- Calculate the empirical and molecular formula of the compound contain...
Text Solution
|
- A compound has the following percentage composition. Carbon 80%, Hydro...
Text Solution
|
- किसी यौगिक में 50% कार्बन, 50% ऑक्सीजन तथा इसका अणुभार लगभग 290 है। इस...
Text Solution
|