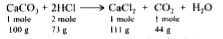Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- 200 grams of marble chips are dropped into 100 g of hydrochloric acid....
Text Solution
|
- Why is not sulphuric acid used for the preparation of CO(2) from marbl...
Text Solution
|
- If 100 grams of calcium carbonate (whether in the form of marble or ...
Text Solution
|
- A sample of hydrochloric acid contains 20 % hydrochloric acid by weigh...
Text Solution
|
- How much marble of 96.5% purity would be required to prepare 10 litres...
Text Solution
|
- (a) What is activation energy? (b) Powdered calcium carbonate reacts...
Text Solution
|
- 10 ग्राम कैल्सियम कार्बोनेट को N. T. P. पर गरम करने पर कितने लीटर कार्...
Text Solution
|
- अभिक्रिया की सन्तुलित रासायनिक समीकरण लिखिए- कैल्सियम कार्बोनेट + हा...
Text Solution
|
- Powdered …………….reacts more quikly with hydrochloric acid than marble c...
Text Solution
|