A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
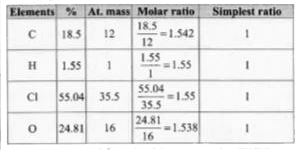
- If a compound, on analysis was found to contain C= 18.5%, H = 1.55%, C...
Text Solution
|
- A compound (60 gm) on analysis gave C=24gm H=4gm and O=31gm , its empi...
Text Solution
|
- If a compound on analysis was found to contain C=18.5%, h=1.55%,Cl=55....
Text Solution
|
- An organic compound contains C, H, and O. Its elemental analysis gave ...
Text Solution
|
- A compound on analysis was found to contain C = 34.6 %, H = 3.85 % and...
Text Solution
|
- An organic compound is found to contain C=40.0%,H=6.66%.The empirical ...
Text Solution
|
- If a compound on analysis was found to contain C = 18.5 H = 1.55% ,Cl=...
Text Solution
|
- A compound (80 g) on analysis gave C=24 g, H=4 g, O=32 g. Its empirica...
Text Solution
|
- 60g of a compound on analysis gave C=24g, H=4g and O=32g. Its empirica...
Text Solution
|