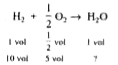A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If 10 volume of dihydrogen gas reacts with 5 volume of dioxygen gas, h...
Text Solution
|
- If ten volumes of dihydrogen gas reats with five volumes of dioxygen g...
Text Solution
|
- Law pf combining volumes: if ten volumes of dihydrogen gas react wit...
Text Solution
|
- यदि डाइनाइट्रोजन गैस के 10 आयतन डाइ ऑक्सीजन गैस के 5 आयतनों से सा...
Text Solution
|
- यदि डाईहाइड्रोजन गैस के 10 आयतन डाइऑक्सिजन गैस के 5 आयतनों के साथ अभिक...
Text Solution
|
- If ten volumes of dihydrogen gas reacts with five volumes of dioxygen ...
Text Solution
|
- If ten volumes of dihydrogen gas reacts with five volumes of dioxygen ...
Text Solution
|
- Vapour density of a gas is 11.2 . What is the volume of 10 g of that g...
Text Solution
|
- If ten volumes of dihydrogen gas react with five volumes of dioxygen g...
Text Solution
|