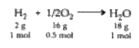A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 10 g of hydrogen and 32 g of oxygen were filled in a steel vessel and ...
Text Solution
|
- 10 g of hygrogen and 64 g of oxygen were filled in a steel vessel and ...
Text Solution
|
- 10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and ...
Text Solution
|
- 10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and ...
Text Solution
|
- 10 g of hydrogen and 64 g of oxygen were filled in a steed vessel and ...
Text Solution
|
- Minimum m.w. rarr molecule at least contain one selenium. 10 g of hydr...
Text Solution
|
- 10g hydrogen and 64 g oxygen were filled in a steel vessel and explode...
Text Solution
|
- 10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and ...
Text Solution
|
- 10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and ...
Text Solution
|