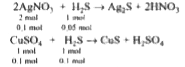A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The ratio of amounts of H2 S needed to precipitate all the metal ions ...
Text Solution
|
- The ratio of amounts of H(2)S needed to precipitate all the metal ions...
Text Solution
|
- The ratio of the molar amounts of H(2)S needed to precipitate the meta...
Text Solution
|
- The ratio of amounts of H(2)S needed to precipitate all the metal ions...
Text Solution
|
- The ratio of the molar amounts of H2S needed to precipitate the metal ...
Text Solution
|
- On treatment of 100 mL of 0.1 M solution of CoCl3.6H2O with excess AgN...
Text Solution
|
- The ratio of amounts of H(2)S needed to precipitate all the metal ions...
Text Solution
|
- The ratio of the molar amounts of H(2)S needed to precipitate the meta...
Text Solution
|
- On treatment of 100 mL of 0.1 M solution of CoCl3.6H2O with excess AgN...
Text Solution
|