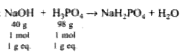A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The equivalent weight of phosphoric acid (H3 PO4) in the reaction, ...
Text Solution
|
- Which of the following acts as reducing agent and why ? H3 PO3 or H3 P...
Text Solution
|
- Basicity of H3 PO4 and H3 PO3 are
Text Solution
|
- The basicity of H3 PO4 is
Text Solution
|
- There is a little difference in acid strength in the series H3 PO4, H3...
Text Solution
|
- There is very little difference in acid strength in the series H3 PO4,...
Text Solution
|
- The equivalent weight of phosphoric acid (H3 PO4 ) in the reaction
Text Solution
|
- The true statement of the acids of phosphorus H3 PO2, H3 PO2 and H3 PO...
Text Solution
|
- For H3 PO3 and H3 PO4, the correct choice is
Text Solution
|