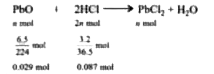A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- How many moles of lead (II) chloride will be formed from a reaction be...
Text Solution
|
- How many moles of lead (II) choride will be formed from a reaction bet...
Text Solution
|
- How many moles of lead (II) chloride will be formed from a reaction be...
Text Solution
|
- How many moles of lead (II) chloride will be formed from a reaction be...
Text Solution
|
- How many moles of lead (II) chloride are formed from a reaction betwee...
Text Solution
|
- How many moles of lead (II) chloride will be formed from a reaction be...
Text Solution
|
- How many moles of lead (II) chloride will be formed from a reaction be...
Text Solution
|
- How many moles of lead (II) chloride will be formed from a reaction be...
Text Solution
|
- 6.5 g PbO तथा 3.2 g HCl की अभिक्रिया से लैड (II) क्लोराइड के कितने मो...
Text Solution
|