A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
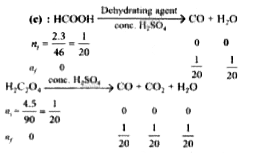
- A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with c...
Text Solution
|
- A mixture of formic acid and oxalic acid is heated with conc. H(2)SO(4...
Text Solution
|
- 1.67 g mixture of Al and Zn was completely dissolved in acid and evolv...
Text Solution
|
- A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with c...
Text Solution
|
- When formic acid or oxalic acid is treated with conc. H(2)SO(4) , the ...
Text Solution
|
- A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with c...
Text Solution
|
- 2.3 g फॉर्मिक अम्ल तथा 4.5 g ऑक्जेलिक अम्ल के एक मिश्रण की सान्द्र H(2...
Text Solution
|
- A mixture of 2.3 g formic acid annd 4.5 g oxalic acid is treated with ...
Text Solution
|
- A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with c...
Text Solution
|