A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE d-AND f- BLOCK ELEMENTS
MTG GUIDE|Exercise CHECK YOUR NEET VITALS|10 VideosTHE d-AND f- BLOCK ELEMENTS
MTG GUIDE|Exercise CHECK YOUR NEET VITALS|10 VideosSURFACE CHEMISTRY
MTG GUIDE|Exercise AIMPT/NEET MCQS|11 VideosTHE p-BLOCK ELEMENTS (GROUP 15 TO 18)
MTG GUIDE|Exercise CHECK YOUR NEET VITALS|14 Videos
Similar Questions
Explore conceptually related problems
MTG GUIDE-THE d-AND f- BLOCK ELEMENTS-NEET CAFE (TOPICWISE PRACTICE QUESTIONS)
- When KCN is added to CuSO4 solution
Text Solution
|
- When KI (excess) is added to I. CuSO4 " " II. HGCl2 " " III. Pb...
Text Solution
|
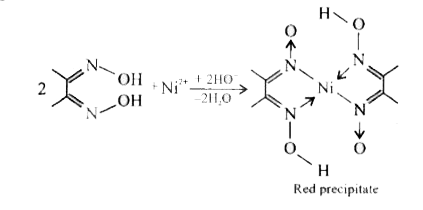
- Ni^(2+) in traces can be tested using
Text Solution
|
- Ti^(2+) is purple while Ti^(4+) is colourless because
Text Solution
|
- Iron is obationed on a commercial scale from Fe2O3 by reduction with
Text Solution
|
- Which of the following has highest melting point?
Text Solution
|
- Anhydrous ferric chloride is prepared by
Text Solution
|
- Which of the following is the most impure form of iron?
Text Solution
|
- An excess of Na2S2O3 reacts with aqueous CuSO4 to give
Text Solution
|
- A colourless solution contains a metal nitrate. A little solution of s...
Text Solution
|
- In which of the following cases, the stability of two oxidation states...
Text Solution
|
- The magnetic moment (B.M) of Fe^(2+) ion is
Text Solution
|
- Amongst TiF(6)^(2-), CoF(6)^(3-) , Cu(2)Cl2 and NiCl4^(2-) (at. Nos. T...
Text Solution
|
- In the aqueous solution, Cu(+1) salts are unstable because
Text Solution
|
- In context with the transition elements, which of the following statem...
Text Solution
|
- Which of the following dissolves in hot concentrated NaOH solution?
Text Solution
|
- Native silver metal forms a water soluble complex with a dilute aqueou...
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
- AgCl dissolves in ammonia solution giving
Text Solution
|
- Copper cannot replace ....... from solution.
Text Solution
|