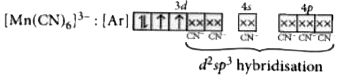
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG GUIDE-COORDINATION COMPOUNDS-CHECK YOUR NEET VITALS
- Match the entries of Column I with appropriate entries of Column II an...
Text Solution
|
- The complex which involves outer orbital hybridisation of central meta...
Text Solution
|
- Effective atomic number (EAN) of Fe in brown ring complex [Fe(H2O)5NO]...
Text Solution
|
- Which of the following statements is correct about the given complex?
Text Solution
|
- Which of these statements about [Co(CN)6]^(3-) is true?
Text Solution
|
- The hybridisation involved in complex [Ni(CN)4]^(2-) is (At. No. Ni =...
Text Solution
|
- The name of complex ion, [Fe(CN)6]^(3-) is
Text Solution
|
- The sum of coordination number and oxidation number of the metal M in ...
Text Solution
|
- Number of possible isomers for the complex [Co("en")2Cl2]Cl will be (...
Text Solution
|
- Which of the following has longest C-O bond length? (Free C - O bond l...
Text Solution
|
- The correct increasing order of trans-effect of the following species ...
Text Solution
|
- Jahn-Teller effect is not observed in high spin complexes of
Text Solution
|
- An example of a sigma bonded organometallic compound is
Text Solution
|
- The correct order of the stoichiometries of AgCl formed when AgNO3 in...
Text Solution
|
- Correct increasing order for the wavelengths of absorption in the visi...
Text Solution
|
- Pick out the correct statement with respect to [Mn(CN)6]^(3-)
Text Solution
|
- The type of isomerism shown by the complex [CoCl("en")2] is
Text Solution
|
- The geometry and magnetic behaviour of the complex [Ni(CO)4] are
Text Solution
|
- Iron carbonyl, Fe(CO)5 is
Text Solution
|
- What is the correct electronic configuration of the central atom in K4...
Text Solution
|