Similar Questions
Explore conceptually related problems
Recommended Questions
- The following results have been obtained during the kinetic studies of...
Text Solution
|
- A following mechanism has been proposed for a reaction 2A +B rarr D ra...
Text Solution
|
- For a reaction 2A +B rarr C+D, the active mass of B is kept constant b...
Text Solution
|
- During the kinetic study of the reaction, 2A + B to C + D. Following r...
Text Solution
|
- Consider the reaction 2A + B to C + D Following results was obtained i...
Text Solution
|
- The following initial rate data were obtained at 300K for the reaction...
Text Solution
|
- During the kinetic study of the reaction, 2A+BtoC+D , following result...
Text Solution
|
- 2A + B rarr C + D अभिक्रिया की बलगतिकी अध्ययन करने पर निम्नलिखित परिणा...
Text Solution
|
- The following results have been obtained during the kinetic studies of...
Text Solution
|
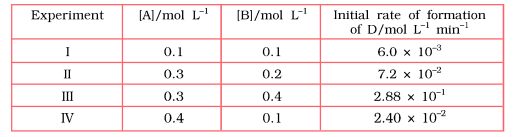 Determine the rate law and the rate constant for the reaction.
Determine the rate law and the rate constant for the reaction.