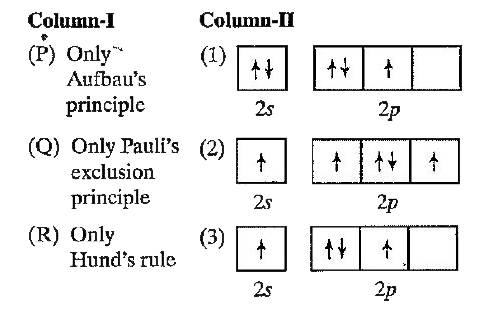
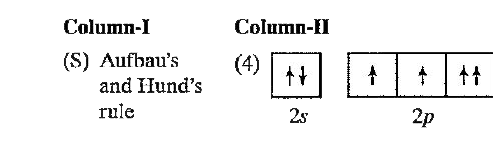
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Match the electronic configuration with the rule that it is violating.
Text Solution
|
- Statement : Aufbau rule is violated in writing electronic configuratio...
Text Solution
|
- The electronic configuration in the valence shell of silicon is . The ...
Text Solution
|
- The electronic configureation in which Pauli's exclusion principal or ...
Text Solution
|
- Assertion. Aufbau rule is violated in writing electronic configuration...
Text Solution
|
- Which rule is violated in the electronic configuration 1s^(0) 2 s^(2) ...
Text Solution
|
- Which rule is violated in the electronic configuration 1s^(0) 2s^(2) 2...
Text Solution
|
- Which rule is violated in the following electronic configuration?
Text Solution
|
- Cr तथा Cu के इलेक्ट्रॉनिक विन्यास लिखिए तथा इनमें ऑफबौ नियम के उल्लंघन...
Text Solution
|