Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-THERMODYNAMICS-Long Answer Questions
- State and explain the Hess's law of constant heat summation.
Text Solution
|
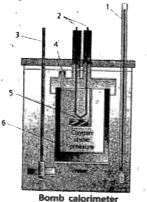
- Explain the experiment to determine the internal energy change of a ch...
Text Solution
|
- Explain the experiment to determine the enthalpy change of a chemical ...
Text Solution
|
- Explain the spontaneity of a reaction in terms of enthalpy change, ent...
Text Solution
|