Text Solution
Verified by Experts
Topper's Solved these Questions
MARCH - 2016 (ANDHRA PRADESH)
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SECTION C |3 VideosMARCH - 2016 (ANDHRA PRADESH)
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SECTION C |3 VideosHYDROGEN AND ITS COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise IMPORTANT QUESTIONS|21 VideosMARCH - 2016 (TELANGANA)
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SECTION C |3 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-MARCH - 2016 (ANDHRA PRADESH)-SECTION B
- Write the postulates of kinetic molecular theory of gases .
Text Solution
|
- Explain the structure of diborane.
Text Solution
|
- What is Hard water ? How can you remove hardness of water by Calgon me...
Text Solution
|
- Explain different types of hydrogen bonds with examples.
Text Solution
|
- What is conjugate Acid -Base pair ? Write the conjugate acid and conju...
Text Solution
|
- Compare diploment of NH(3) molecule with that of NF(3) molecule .
Text Solution
|
- Discuss the various reactions that occur in the Solvay process.
Text Solution
|
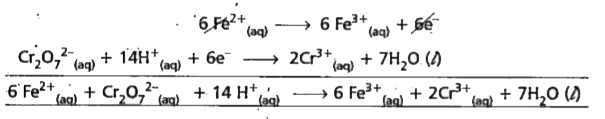
- Balance the following equation in acid medium by Ion-electron method :...
Text Solution
|