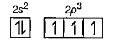A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Maximum number of covalent bonds formed by N and P are
Text Solution
|
- The number of covalent bonds formed by beryllium is
Text Solution
|
- Maximum number of covalent bonds formed by N and P are
Text Solution
|
- नाइटूजिन के द्वारा बनने वाले सहसंयोजक आबन्धों की संख्या अधिकतम 4 ही क्...
Text Solution
|
- Molecule having maximum number of covalent bonds is
Text Solution
|
- सहसंयोजी यौगिकों में दो परमाणुओं के मध्य अधिकतम सहसंयोजी बन्धों की संख...
Text Solution
|
- सहसंयोजी यौगिकों में दो परमाणुओं के मध्य अधिकतम् सहसंयोजी बन्धों की सं...
Text Solution
|
- सहसंयोजी यौगिकों में दो परमाणुओं के मध्य अधिकतम सहसंयोजी बन्धों की संख...
Text Solution
|
- सहसंयोजी यौगिकों में दो परमाणुओं के मध्य अधिकतम सहसंयोजी बन्धों की संख...
Text Solution
|