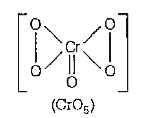
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When H(2)O(2) is added to an acidified K(2)Cr(2)O(7) solution
Text Solution
|
- When H(2)S is passed through acidified K(2)Cr(2)O(7) solution, the sol...
Text Solution
|
- When acidified K(2)Cr(2)O(7) solution is added to Sn^(2+) salts then S...
Text Solution
|
- Acidified solution of K(2)Cr(2)O(7) on treatment with H(2)O(2) yields ...
Text Solution
|
- What happens when H(2)O(2) is shaken with acidified K(2)Cr(2)O(7) solu...
Text Solution
|
- When H(2)O(2) is added to a acidified solution of K(2)Cr(2)O(7) :
Text Solution
|
- Acidified K(2)Cr(2)O(7) reacts with H(2)O(7) to give "…............." ...
Text Solution
|
- When acidified K(2)Cr(2)O(7) solution is added to Sn^(2+) salts then S...
Text Solution
|
- Acidified solution of K(2)Cr(2)O(7) on treatment with H(2)O(2) yields ...
Text Solution
|