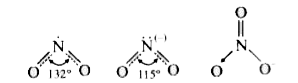
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The ONO bond angle is maximum in
Text Solution
|
- The ONO angle is maximum in :
Text Solution
|
- The ONO bond angle is maximum in
Text Solution
|
- The ONO bond angle is maximum in
Text Solution
|
- Maximum bond angle between two bond is in molecule.
Text Solution
|
- The ONO angle is maximum in-
Text Solution
|
- दो सहसंयोजी बन्धो के बीच बन्ध कोण अधिकतम है
Text Solution
|
- Which of the following has largest ONO bond ?
Text Solution
|
- কোন্ অণু/আয়নটিতে ONO বন্ধন-কোণের মান সর্বোচ্চ-
Text Solution
|