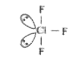
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the structure of ClF3, the number of lone pairs of electrons on cen...
Text Solution
|
- In the structure of ClF(3), the number of lone pairs of electrons on c...
Text Solution
|
- In the structure of CIF(3) , the number of lone pair of electrons on c...
Text Solution
|
- The number of lone pair of electrons in central atom of CIF5
Text Solution
|
- In the structure of ClF(3) , the number of lone pairs of electrons on ...
Text Solution
|
- In the structure of CIF(3) , the number of lone pairs of electrons...
Text Solution
|
- In the structure of CIF(3) , the number of lone pairs of electrons on ...
Text Solution
|
- Give oxidation state and number of lone pair of electron in CLF3
Text Solution
|
- In the structure of CIF3 , the number of lone pairs of electrons on ce...
Text Solution
|