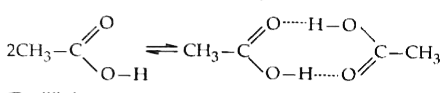A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is correct for the given equilibrium? (Equ...
Text Solution
|
- For A+BhArrC+D , the equilibrium constant is K(1) and for C+DhArA+B, ...
Text Solution
|
- For the hypothetical reactions, the equilibrium constant (K) value are...
Text Solution
|
- Equilibrium constants for four different reaction are given as K(1)=10...
Text Solution
|
- For the rections AhArrB,BhArrC,ChArrD,, equilibrium constants are K(1)...
Text Solution
|
- For a gaseous reaction 2BtoA ,the equilibrium constant K(p) is ……to/th...
Text Solution
|
- For the hypothetic reaction, the equilibrium constant (K) values are g...
Text Solution
|
- The relationship between equilibrium constants K(p) "and" K(c) for a g...
Text Solution
|
- स्थिर ताप पर किसी अभिक्रिया के लिये साम्य स्थिरांक K(p) व K(c ) का सम...
Text Solution
|