Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK GLOBAL PUBLICATION-MODEL QUESTION PAPER-6-SECTION-B
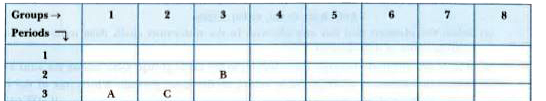
- The position of three elements A, B and C in the Periodic Table is sho...
Text Solution
|
- Which of these can be used to produce a colourless and odourless gas w...
Text Solution
|
- A student adds a spoon full of powdered sodium hydrogen carbonate to a...
Text Solution
|
- Why is some KOH placed in a small test tube in the flask with germina...
Text Solution
|
- The rest positions of the needles in a milliammeter and voltmeter not ...
Text Solution
|