Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
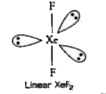
- Explain the following situations : XeF(2) has a straight linear str...
Text Solution
|
- If one assume linear structure instead of bent structure for water the...
Text Solution
|
- XeF(2) has linear structure and not a bent structure , Given reason .
Text Solution
|
- Water molecule is bent and not linear in structure. Explain.
Text Solution
|
- Explain the following situations : (i) In the structure of HNO(3) mole...
Text Solution
|
- Explain the structures of XeF(2)
Text Solution
|
- Explain the structures of XeF(4)
Text Solution
|
- XeF(2) तथा XeF(4) की संरचना समझाइये|
Text Solution
|
- XeF(2) has straight linear structure and not a bent structure.
Text Solution
|