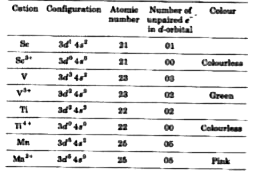
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following cations are coloured in aqueous solutions and w...
Text Solution
|
- Which one of the following forms a colourless solution in aqueous medi...
Text Solution
|
- (a) Which metal in the first transition series (3d series) exhibits+ 1...
Text Solution
|
- Predict which of the following will be coloured in aqueous solution ? ...
Text Solution
|
- (a) Which metal in the first transition series (3d series) exhibits +1...
Text Solution
|
- Predict which of the following will be coloured in aqueous solution ?...
Text Solution
|
- Which of the following cations are coloured in aqueous solution and wh...
Text Solution
|
- Sc^(3+) is colourless is aqueous solution whereas Ti^(3+) is coloured.
Text Solution
|
- The aqueous solution containing which one of the following ions will b...
Text Solution
|