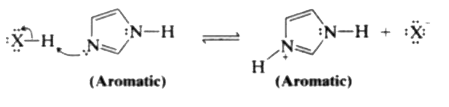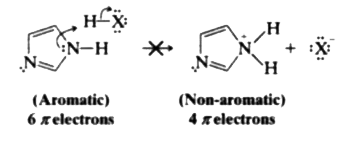Text Solution
Verified by Experts
Topper's Solved these Questions
AROMATIC COMPOUNDS
MS CHOUHAN|Exercise Additional Objective Questions (Single Correct Choice Type)|20 VideosAROMATIC COMPOUNDS
MS CHOUHAN|Exercise Additional Objective Questions (Linked Comprehension Type)|6 VideosAROMATIC COMPOUNDS
MS CHOUHAN|Exercise Subjective Problems|3 VideosAN INTRODUCTION TO ORGANIC REACTIONS AND THEIR MECHANISMS ACIDS AND BASES
MS CHOUHAN|Exercise ADDITIONAL QUESTION (SINGLE CORRECT CHOICE TYPE )|32 VideosBIOMOLECULES
MS CHOUHAN|Exercise Level 2|1 Videos
Similar Questions
Explore conceptually related problems
MS CHOUHAN-AROMATIC COMPOUNDS-Solved Problem
- Provide a name for each of the following compounds.
Text Solution
|
- Using the polygon-and-circle method to outline the molecular orbitals ...
Text Solution
|
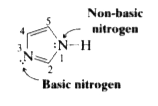
- Imidazole (at right) has two nitrogens, N3 is relatively basic (like t...
Text Solution
|
- Designate whether each of the following compounds is aromatic, non-aro...
Text Solution
|
- Designate whether each of the following compounds is aromatic, non-aro...
Text Solution
|
- Designate whether each of the following compounds is aromatic, non-aro...
Text Solution
|
- Designate whether each of the following compounds is aromatic, non-aro...
Text Solution
|
- Designate whether each of the following compounds is aromatic, non-aro...
Text Solution
|
- Designate whether each of the following compounds is aromatic, non-aro...
Text Solution
|
- Designate whether each of the following compounds is aromatic, non-aro...
Text Solution
|
- Designate whether each of the following compounds is aromatic, non-aro...
Text Solution
|
- Designate whether each of the following compounds is aromatic, non-aro...
Text Solution
|
- In the given compound, will Br ionize in the form of (a) Br^(-) or (b)...
Text Solution
|
- The given compound has high dipole moment. Explain.
Text Solution
|
- Explain why the given compound undergoes dimerization at room temperat...
Text Solution
|
- Compare the rate of reaction of the following compounds with AgNO(3).
Text Solution
|
- Classify each as aromatic, anti-aromatic and non-aromatic compound.
Text Solution
|
- Classify each as aromatic, anti-aromatic and non-aromatic compound.
Text Solution
|
- Classify each as aromatic, anti-aromatic and non-aromatic compound.
Text Solution
|
- Classify each as aromatic, anti-aromatic and non-aromatic compound.
Text Solution
|