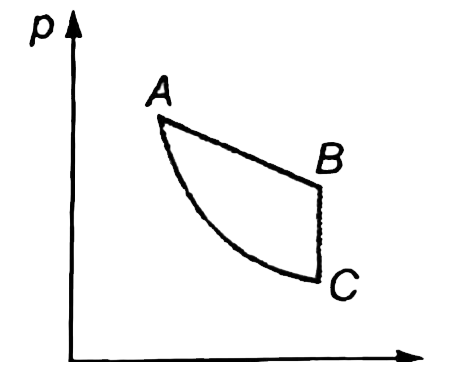
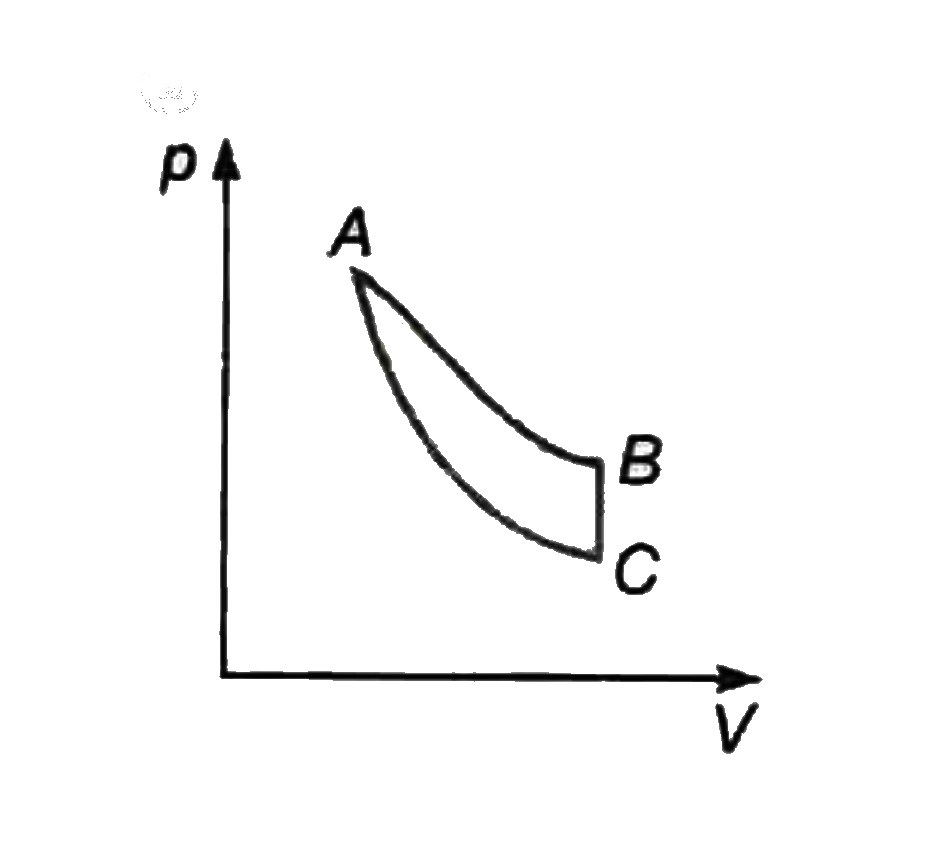
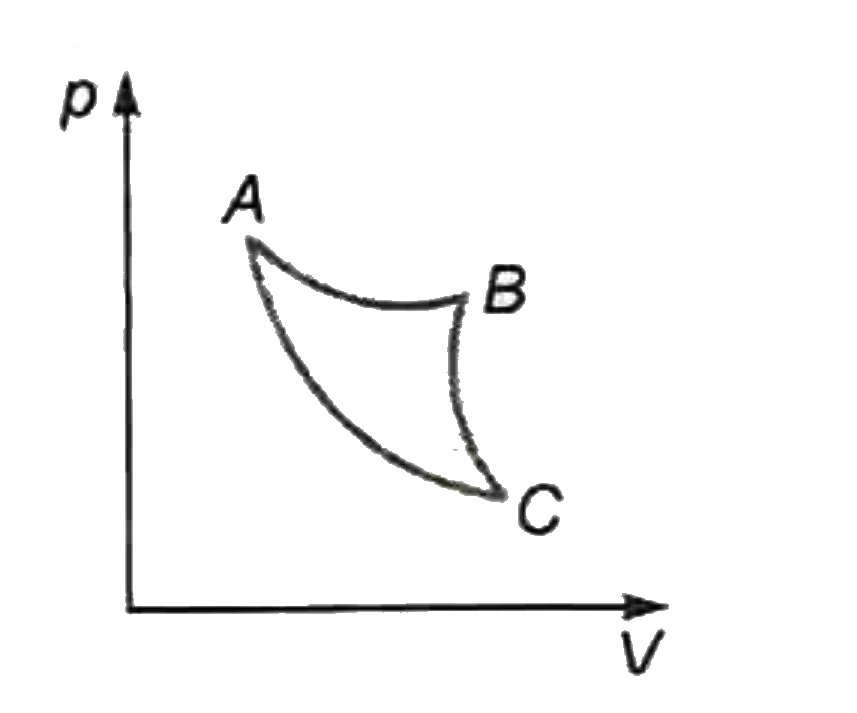
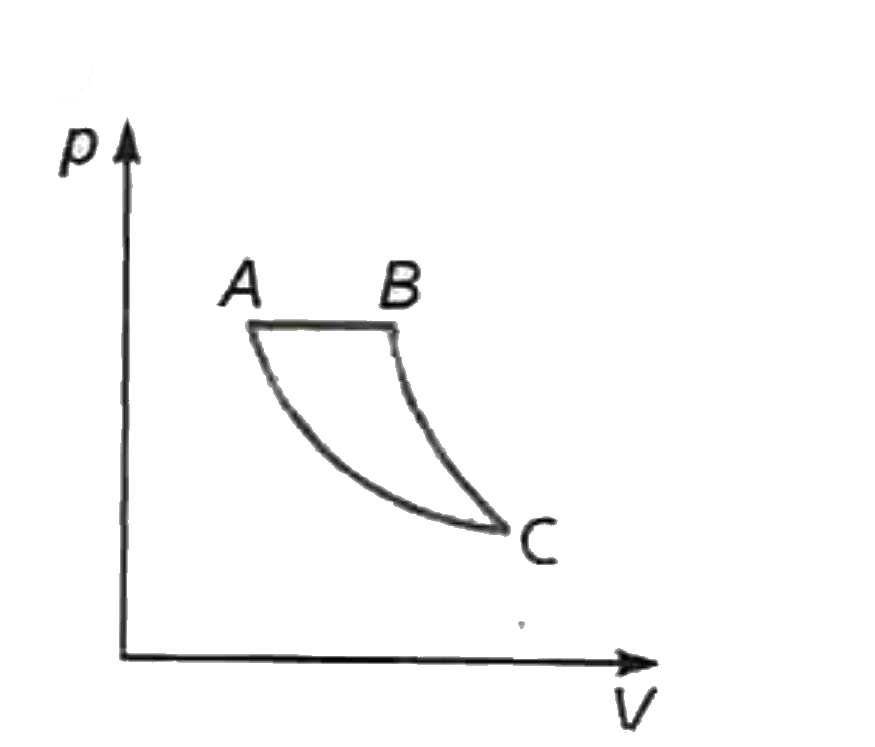
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If AB is an isothermal BC is an isochoric and AC is an adiabatic, whi...
Text Solution
|
- The pressure v//s volume graph of an ideal gas is given different ther...
Text Solution
|
- The curves A and B in the figure shown P-V graphs for an isothermal an...
Text Solution
|
- In which of the following indicator diagrams gives below do AB,BC , an...
Text Solution
|
- In the given graph, adiabatic and isothermal curves are shown. Then,
Text Solution
|
- दी गई आकृति में, DB bot BC, DE bot AB, AC bot BC है तो सिद्ध कीजिए कि...
Text Solution
|
- If AB is an isothermal BC is an isochoric and AC is an adiabatic, whic...
Text Solution
|
- If DA and BC are adiabatic curves and AB and CD are isothermal curves ...
Text Solution
|
- What are isobaric, isochoric, isothermal and adiabatic processes?
Text Solution
|