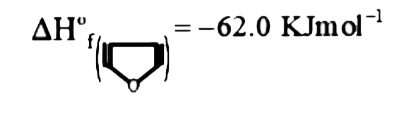Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate heat of atomization of furan using the data Heats of ato...
Text Solution
|
- Using the bond enthalpy data given below, calculate the enthalpy of fo...
Text Solution
|
- Calculate the enthalpy of combustion of methyl alcohol at 298 K from t...
Text Solution
|
- Compute the heat of formation of liquid methyl alcohol in kJ mol^(-1) ...
Text Solution
|
- Calculate the heat of combustion of eithene CH(2) = CH(2)(g) + 3O(2)...
Text Solution
|
- The heat of combustion of carbon is 394 kJ mol^(-1). The heat evolved ...
Text Solution
|
- Calculate heat of atomization of furan using the data Heat of atomizat...
Text Solution
|
- Calculate the heat of combustion of methyl alcohol at 298 K from the f...
Text Solution
|
- Compute the heat of formation of liquid methyl alcohol in kJ mol^(-1),...
Text Solution
|