Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
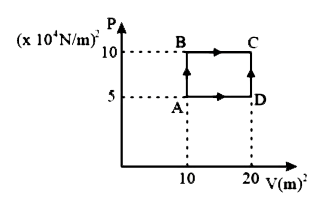
- A sample of 2kg monoatomic helium gas (assumed ideal) is taken through...
Text Solution
|
- Find the average magnitude of linear momentum of a helium molecule in ...
Text Solution
|
- A sample of 2kg of helium (assumed ideal) is taken through the process...
Text Solution
|
- A sample of 2 kg of monatomic helium (assumed ideal) is taken through ...
Text Solution
|
- A sample of 2 kg of monoatomic helium (assumed ideal) is taken through...
Text Solution
|
- A sample of 2 kg of helium (assumed ideal) is taken through the proces...
Text Solution
|
- A sample of ideal gas is taken through the cyclic process shown in the...
Text Solution
|
- A sample of ideal gas is taken through the cyclic process shown in the...
Text Solution
|
- A sample of 2 kg monoatomic helium (assumed ideal ) is taken from A to...
Text Solution
|