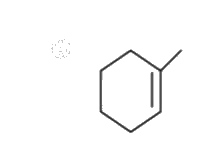
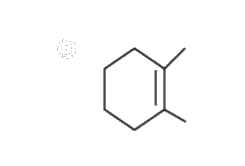
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Hydroboration oxidation and acid hydration will yield the same product...
Text Solution
|
- Hydroboration oxidation and acid hydration will yield the same product...
Text Solution
|
- Acid-catalysed hydration oxymercuration-demercu-ration, and hydroborat...
Text Solution
|
- (HBO) Hydroboration and Oxymercuration-Demercuration, and acid catalys...
Text Solution
|
- Isobutylene when subjected to hydroboration oxidation reaction yields
Text Solution
|
- The hydroboration oxidation of 2-methyl propene yields-
Text Solution
|
- Which of the following alkenes will give same product by any method ou...
Text Solution
|
- Following alkene will give same product by any method out of hydration...
Text Solution
|
- Which of the following alkenes will give same product by any method o...
Text Solution
|