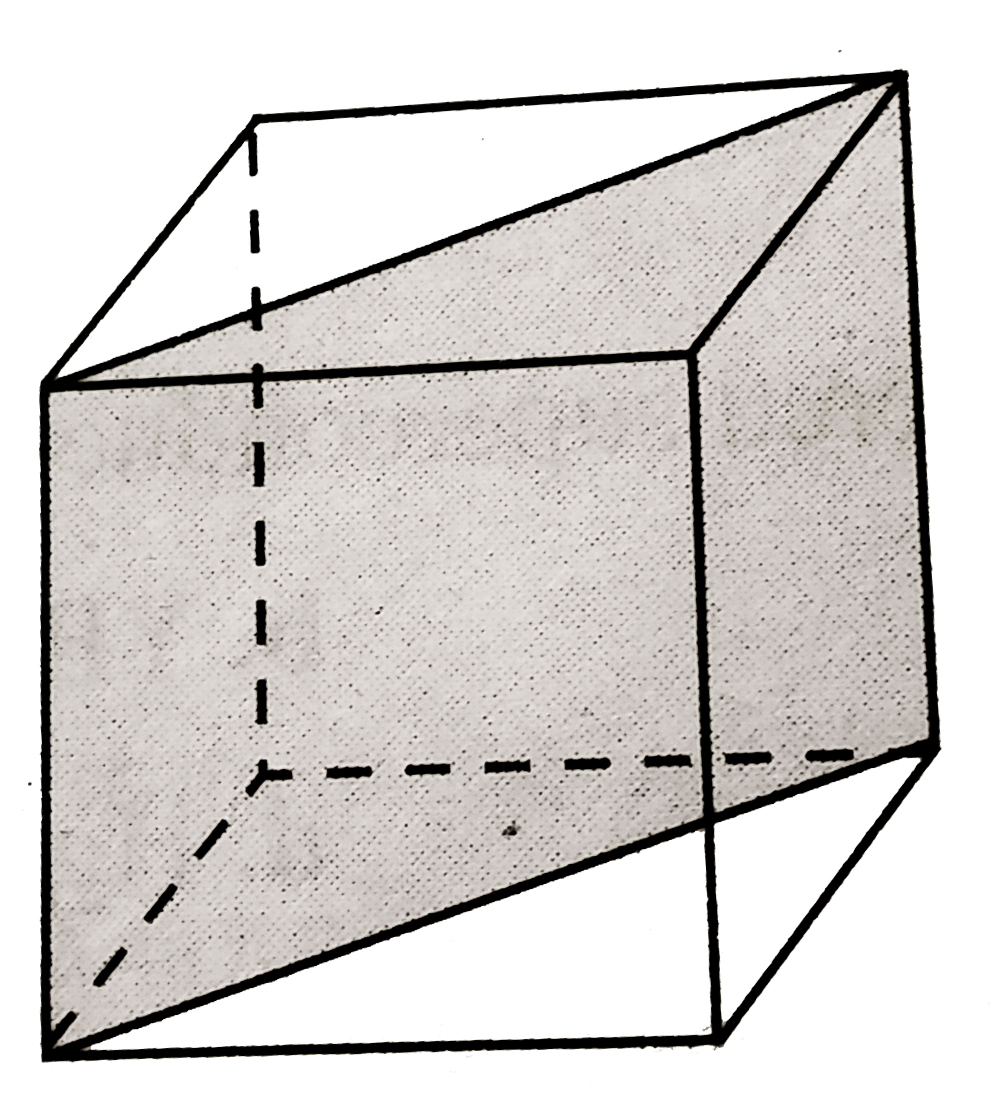
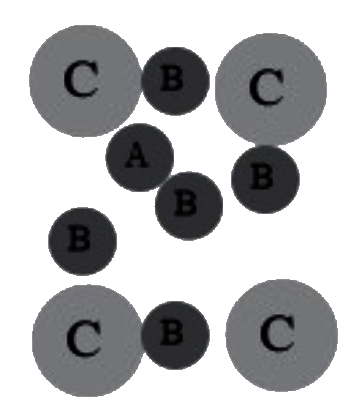
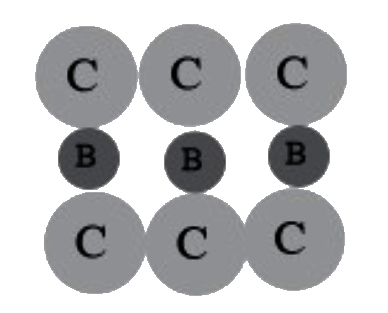
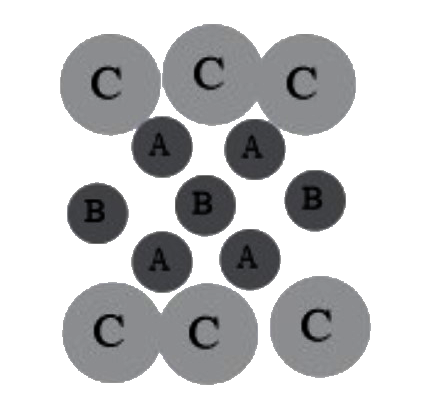
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In a hypothetical solid, C atoms are found to form cubical close-packe...
Text Solution
|
- A forms ccp lattice B occupy half of the octahedral voids and O occupy...
Text Solution
|
- In a hypothetical solid, C atoms are found to form cubical close-packe...
Text Solution
|
- In a hypothetical solid C atoms form C C P lattice with A atoms occupy...
Text Solution
|
- A solid is formed and it has three types of atoms X, Y and Z, X forms ...
Text Solution
|
- In a solid between A and B atoms of A are arranged in ccp array and at...
Text Solution
|
- In a hypothetical solid , C atoms are found to form cubical close - pa...
Text Solution
|
- A solid is formed and it has three types of atoms X, Y and Z, X forms ...
Text Solution
|
- A solid is formed by three elements X,Y and Z, X atoms form a fcc latt...
Text Solution
|