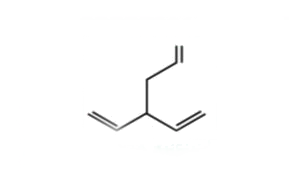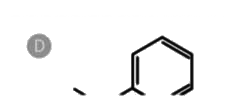A
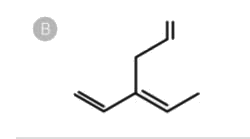
B
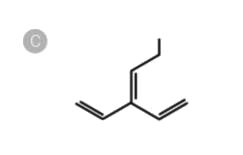
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Highest heat of hydrogenation is shown by which of the following compo...
Text Solution
|
- Which of the following has highest heat of hydrogenation:
Text Solution
|
- Which of the following is havin highest heat of hydrogenation?
Text Solution
|
- Which of the following orders is correct for heat of hydrogenation of ...
Text Solution
|
- Which of the following order is correct for heat of hydrogenation of t...
Text Solution
|
- Which of the following alkene has highest value of heat of hydrogenati...
Text Solution
|
- Which one of the following compounds would have the highest heat of hy...
Text Solution
|
- Which of the following compounds has the highest heat of combustion?
Text Solution
|
- In which compound heat of combustion is highest :
Text Solution
|