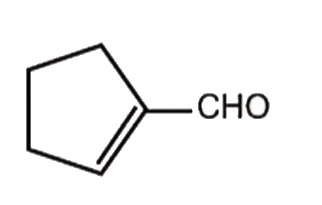
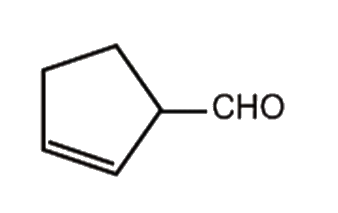
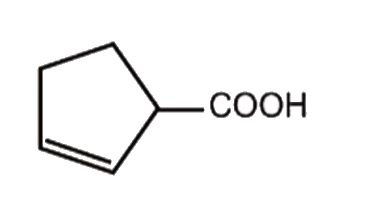
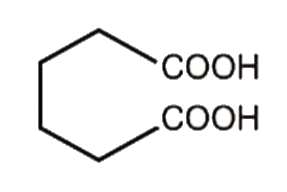
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Cyclohexene on ozonolysis followed by reaction with zinc dust and wate...
Text Solution
|
- An organic compound (E)(C(5)H(8)) , on hydrogenation gives a compound ...
Text Solution
|
- Cyclohexene on ozonolysis followed by reaction with zinc dust and wate...
Text Solution
|
- A compound on dehydrohalogenatio with alcoholic KOH gives alkyne but o...
Text Solution
|
- Compound A on reduction gives B which on further reaction with CHCl(3)...
Text Solution
|
- Cyclohexene on ozonolysis followed by reaction with zinc dust and wate...
Text Solution
|
- निम्नलिखित यौगिकों की जलीय KOh से कराने पर कौन-सा यौगिक dl उत्पाद (रे...
Text Solution
|
- Cyclohexene on ozonolysis followed by reaction with zinc dust and wate...
Text Solution
|
- A compound (A) C(5)H(9)Br on treatment with Mg in dry ether gives a co...
Text Solution
|