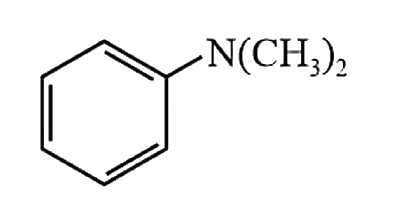
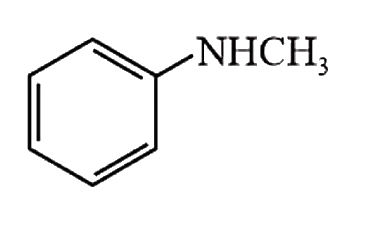
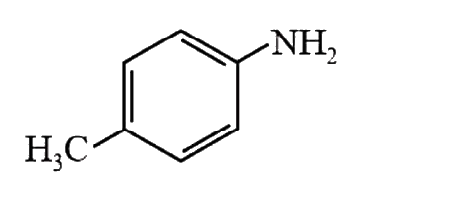
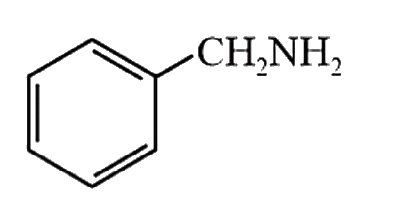
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Amongst the compounds given, the one that would form a brilliant color...
Text Solution
|
- Amongst the compounds gives, the one that would form a brilliant color...
Text Solution
|
- A: An organic compound on diazotisation followed by reaction with alka...
Text Solution
|
- Amongst the compounds given, the one that would form a brilliant colou...
Text Solution
|
- Among the compounds given, the one that would form a brilliant colored...
Text Solution
|
- दिये गए यौगिकों में से कौन सा NaNO(2) व तनु HCl से क्रिया करने के बाद ...
Text Solution
|
- Amongst the compounds given, the one that would form a brilliant color...
Text Solution
|
- दिए गए यौगिकों में, एक तनु HCl में NaNO2 के साथ उपचार के बाद B - नैफ्...
Text Solution
|
- दिए गए यौगिकों में, एक तनु HCl में NaNO2 के साथ उपचार के बाद B - नैफ्...
Text Solution
|