A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
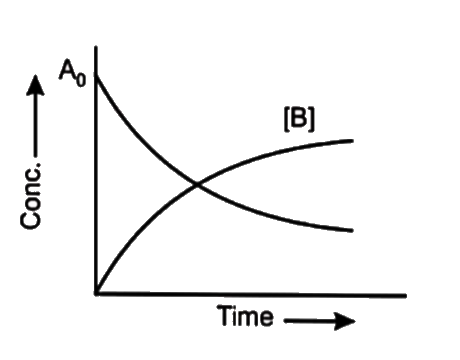
- At the point of intersection of the two curves shown the concentration...
Text Solution
|
- At the point of intersection of the two curves shown, the conc. of B i...
Text Solution
|
- For the reaction A+B rarr C+D The variation of the concentration of th...
Text Solution
|
- At the point of intersection of the two curves shown the concentration...
Text Solution
|
- At the point of intersection of the two curves shown for the reaction ...
Text Solution
|
- At the point of intersection of the two curves shown, the conc.of B is...
Text Solution
|
- For the reaction, A + B to C + D. The variation of the concentration o...
Text Solution
|
- At the given point of intersection of the two curves shown concentrati...
Text Solution
|
- At the point of intersection of the two curves shown the conc. Of B is...
Text Solution
|