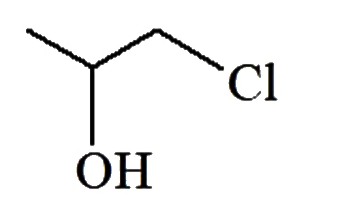
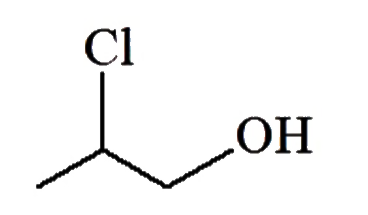
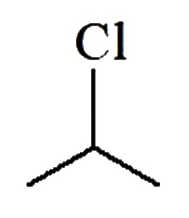
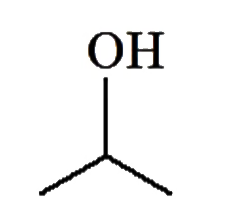
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Propene on reaction with hypochlorous acid gives ?
Text Solution
|
- Hypochlorous Acid
Text Solution
|
- A hydrocarbon reacts with hypochlorous acid to give 2-chloroethanol. T...
Text Solution
|
- Propene on reaction with chlorine water gives
Text Solution
|
- The reaction of chlorine water with propene gives
Text Solution
|
- हाइपोक्लोरस अम्ल की असमानुपातन अभिक्रिया से कौन-से उत्पाद बनते है ?:
Text Solution
|
- हाइपोक्लोरास अम्ल की असमानुपातन (disproportionation) अभिक्रिया के संभा...
Text Solution
|
- हाइपोफ्लोरस अम्ल तथा हाइपोक्लोरस अम्ल के सूत्र तथा संरचनाएँ लिखिए।
Text Solution
|
- Propene on reaction with hypochlorous acid gives ?
Text Solution
|