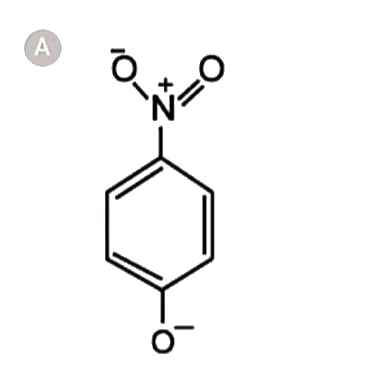
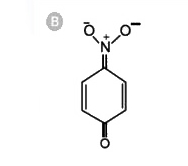
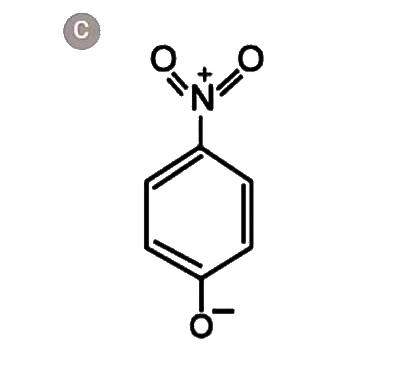
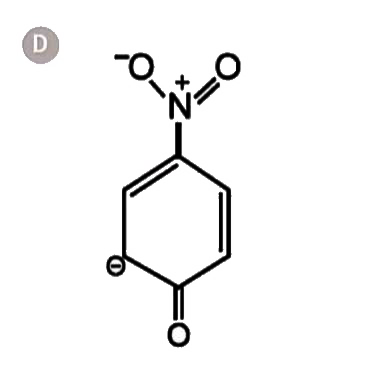
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The most unlikely resonating structures of pnitrophenoxide ion is :
Text Solution
|
- The most unlikely representation of resonance structure of p-nitrophen...
Text Solution
|
- The most unlikely representation of resonance structures of p-nitrophe...
Text Solution
|
- The most unlikely representation of resonance structures of p-nitrophe...
Text Solution
|
- The most unlikely representation of resonance structures of p-nitrophe...
Text Solution
|
- The most unlikely representation of resonance structure of p-nitrophen...
Text Solution
|
- The most unlikely representation of resonance structure of p-nitrophen...
Text Solution
|
- The most unlikely representation of resonance structures of p-nitrophe...
Text Solution
|
- The most unlikely representation of resonance structures of p- ni...
Text Solution
|