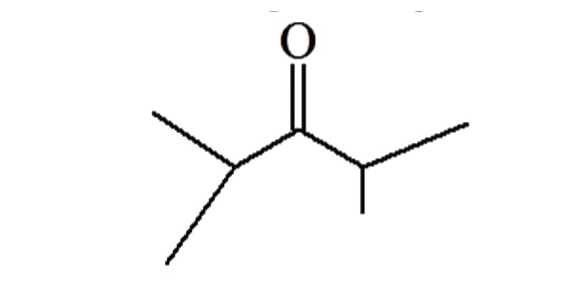
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Number of Deuterium exchange in the given tautomer when the compound i...
Text Solution
|
- Which of the following compound does not undergo base-ctalyzed exchang...
Text Solution
|
- How can D(2)O(2) prepared form water ? Mention the physcial properties...
Text Solution
|
- How can D(2)O(2) prepared form water ? Mention the physcial propert...
Text Solution
|
- कथन-1 -D(2)O भारी जल कहलाता है। कथन-2 -D(2)O हाइड्रोजन के समस्थानिक ...
Text Solution
|
- When phenol is treated with D(2)SO(4)//D(2)O, some of the hydrogens ge...
Text Solution
|
- When phenol is treated with D(2)SO(4)//D(2)O, some of the hydrogens ge...
Text Solution
|
- Number of Deuterium exchange in the given tautomer when the compound i...
Text Solution
|
- Why concentration of D(2) O increases when electrolysis of water is ca...
Text Solution
|