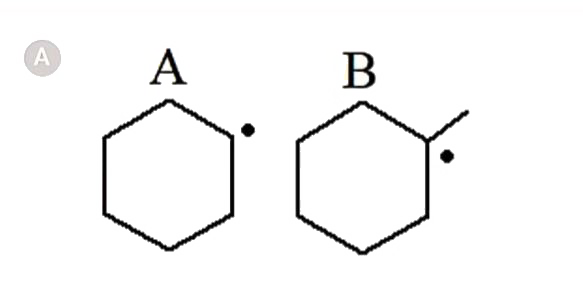
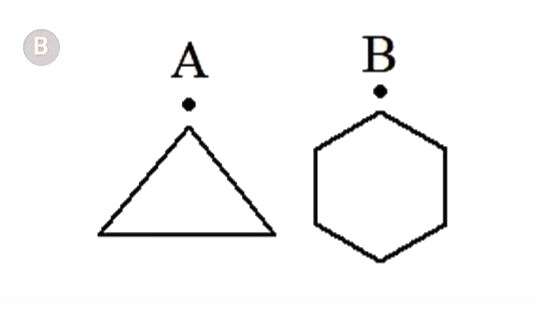
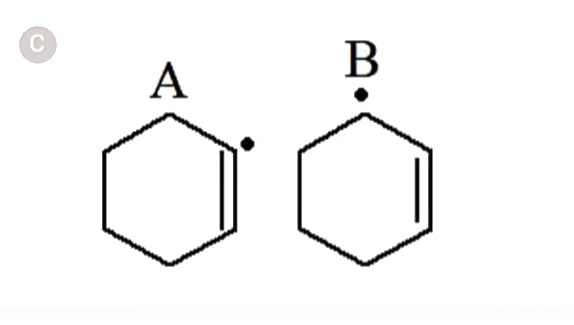
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In which of the following pairs A is more stable than B?
Text Solution
|
- In which of the following pairs II is more stable than I
Text Solution
|
- In which pair second ions is more stable than first ?
Text Solution
|
- In which of the following pairs of carbanion the first one is more sta...
Text Solution
|
- In which of the following pairs A is more stable than B ?
Text Solution
|
- In which of the following pairs A is more stable than B?
Text Solution
|
- In which of the following pairs, first species is more stable than sec...
Text Solution
|
- In which of the following pairs A is more stable than B?
Text Solution
|
- In which of the following pairs A is more stable than B ?
Text Solution
|