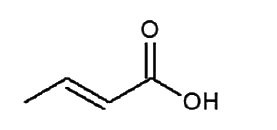
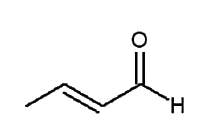
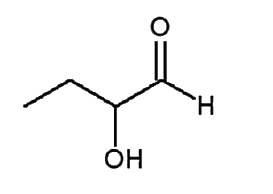
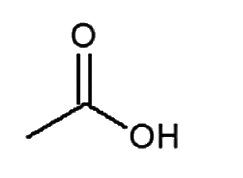
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- H-C-=C-H underset(H(2)SO(4))overset(HgSO(4))(rarr)overset("dil.NaOH")(...
Text Solution
|
- complete the following reactions: a. overset (C(6)H(6) + MeCOCI) un...
Text Solution
|
- 2Ph-overset(O)overset(||)(C)-CH(3)overset(Mg-Hg)underset(H(2)O)rarrove...
Text Solution
|
- The following reaction is an example of H-underset("Ethanol")(underset...
Text Solution
|
- HC-=CHoverset(HgSO(4)//H(2)SO(4))rarr[A]overset("Oxidation")rarr[B]ove...
Text Solution
|
- (i) CHequivCHoverset(H(2)O)underset(H(2)SO(4)//HgSO(4))rarr[A]overset(...
Text Solution
|
- Identify the product S in the following series of operations : C(2)H(2...
Text Solution
|
- In the reaction sequence CH(3)-overset(O)overset(||)(C)-H-underset(.^(...
Text Solution
|
- H-C-=C-H underset(H(2)SO(4))overset(HgSO(4))(rarr)overset("dil.NaOH")(...
Text Solution
|