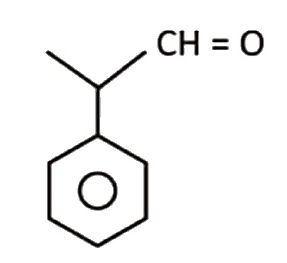
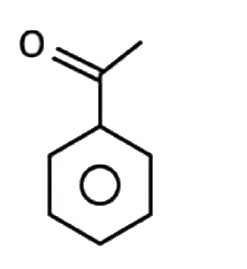
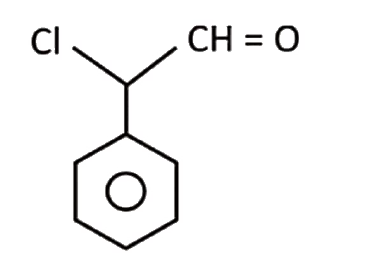
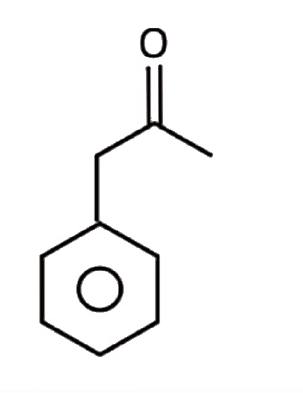
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Poverset(PCl(5),0^(@)C)(rarr)Qunderset(-2HCl (ii)H^(+))overset((i)NaNH...
Text Solution
|
- Complete the following reactions: a. H-underset((A))C-=C-Hoverset(1mol...
Text Solution
|
- CH(3)CH(2)C=Choverset(NaNH(2)//NH(3)(l))rarr overset(CH(3)CH(2)Br)rarr...
Text Solution
|
- CH(3)CH(2)CH(2)Broverset("KOH(alc)")rarr[A]overset(Br(2))rarr[B]unders...
Text Solution
|
- Poverset(PCl(5),0^(@)C)(rarr)Qunderset(-2HCl (ii)H^(+))overset((i)NaNH...
Text Solution
|
- overset((i) NaNH(2), NH(3)(ii)CH(3)-Br)rarr (A) overset((H(2)" Lindlar...
Text Solution
|
- अभिक्रिया में H-C-= CH overset((i) NaNH(2)//liq. NH(3)) underset((ii...
Text Solution
|
- In the reaction : CH(3)-C-=C-Hoverset((i)NaNH(2)//NH(3)(I))rarr(A)ov...
Text Solution
|
- In the reaction H-C-=CHoverset((1)NaNH(2)//liq.NH(3))underset((2)CH(3)...
Text Solution
|