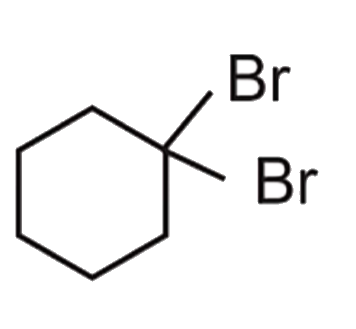
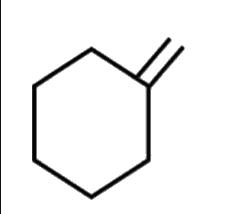
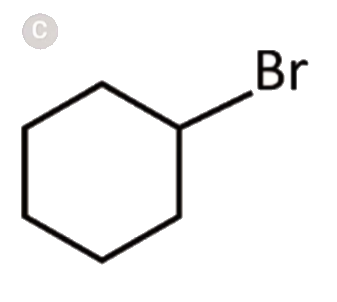
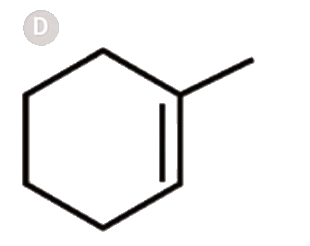
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which reaction produce 1-methylcyclohexene
Text Solution
|
- Which is the correct structure of 4- ethyl 3- methylcyclohexene?
Text Solution
|
- Choose the more stable alkene in each of the following pair. Explain y...
Text Solution
|
- Which of the following is the best stereochemical representation when ...
Text Solution
|
- 1-Methylcyclohexene is allowed to react with B(2)H(6). The product is ...
Text Solution
|
- Which of the following is the best reagent to convert 1-Methylcyclohex...
Text Solution
|
- Select the major product obtained from the addition of HBr to 1-methyl...
Text Solution
|
- Which reaction produce 1-methylcyclohexene
Text Solution
|
- Choose the more stable alkene in each of the following pairs. Explain ...
Text Solution
|