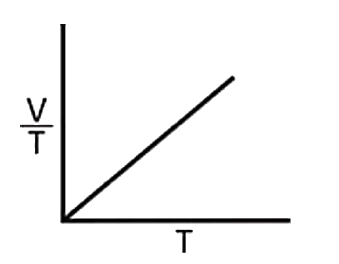
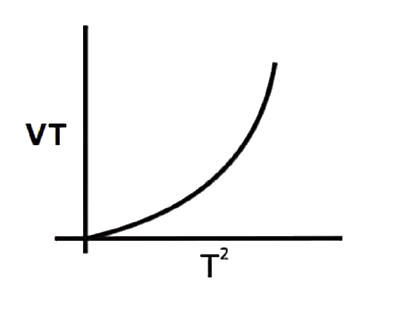
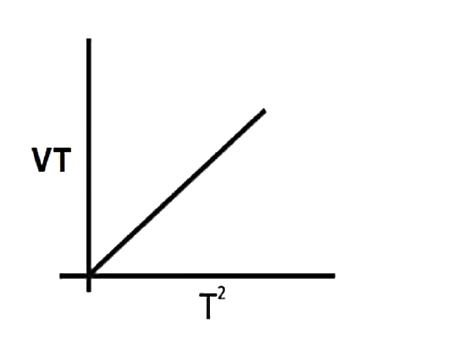
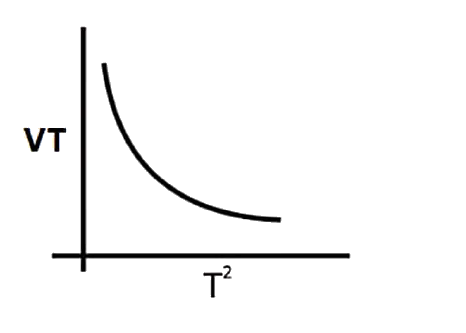
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following curve is correct for a given amount of an ideal...
Text Solution
|
- V vs T curves at constant pressure P(1) and P(2) for an ideal gas are ...
Text Solution
|
- Which of the following curve is correct for an ideal gas ?
Text Solution
|
- When a sample of ideal gas is changed from an initial state to a final...
Text Solution
|
- Which of the following curve is incorrect for given amount of an ideal...
Text Solution
|
- Which of the following curve is correct for a given amount of an ideal...
Text Solution
|
- At a constant pressure, the density of a certain amount of an ideal ga...
Text Solution
|
- Which of the following is a correct plot of the volume of fixed amount...
Text Solution
|
- An ideal gas at constant pressure 10J Works. The amount of heat that i...
Text Solution
|