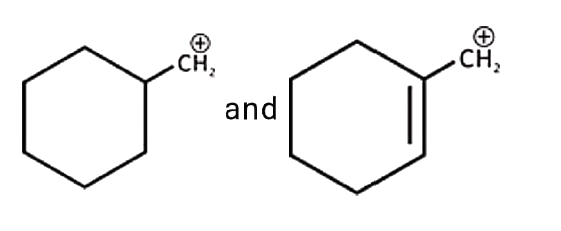
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Some pairs of ions are given below. In which pair, first ion is more s...
Text Solution
|
- In which of the following pairs, the first atom or ion is not large th...
Text Solution
|
- Some pairs of aids are given below. Select the pair in which second ac...
Text Solution
|
- In which pair second ions is more stable than first ?
Text Solution
|
- In which pair, second ion is more stable than first?
Text Solution
|
- In which pair the first atom or ion is not larger than the second :-
Text Solution
|
- किस युग्म में प्रथम परमाणु आयन द्वितीय से बड़ा नहीं है?
Text Solution
|
- In which of the following pairs, first species is more stable than sec...
Text Solution
|
- Which of the following is second ion is more stable than the first
Text Solution
|