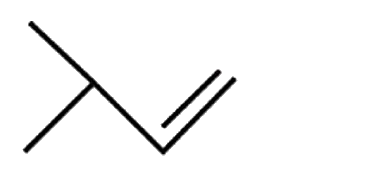
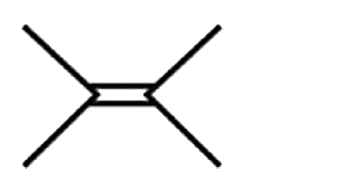
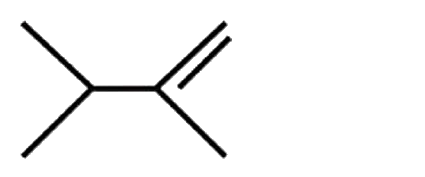
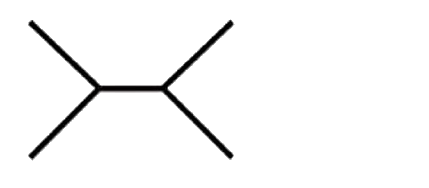
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- CH(3)-underset(CH3)underset(|)overset(CH3)overset(|)(C )-underset(Br)u...
Text Solution
|
- CH(3)-underset(CH3)underset(|)overset(CH3)overset(|)(C )-underset(Br)u...
Text Solution
|
- Major product of the given reaction is- CH(3)-underset(H3C)underset(|)...
Text Solution
|
- CH3-overset(CH3)overset(|)underset(OH)underset(|)C-overset(CH3)overset...
Text Solution
|
- The product of the given reaction , CH3 - underset( OH )underse...
Text Solution
|
- CH(3) - underset(CH(3))underset(|)overset(CH(3))overset(|)(C ) - Br un...
Text Solution
|
- The product of the given reaction , CH3 - underset( OH )underse...
Text Solution
|
- CH(3)-underset(CH3)underset(|)overset(CH3)overset(|)(C )-underset(Br)u...
Text Solution
|
- What would be the major products in the following reactions: CH3 - u...
Text Solution
|