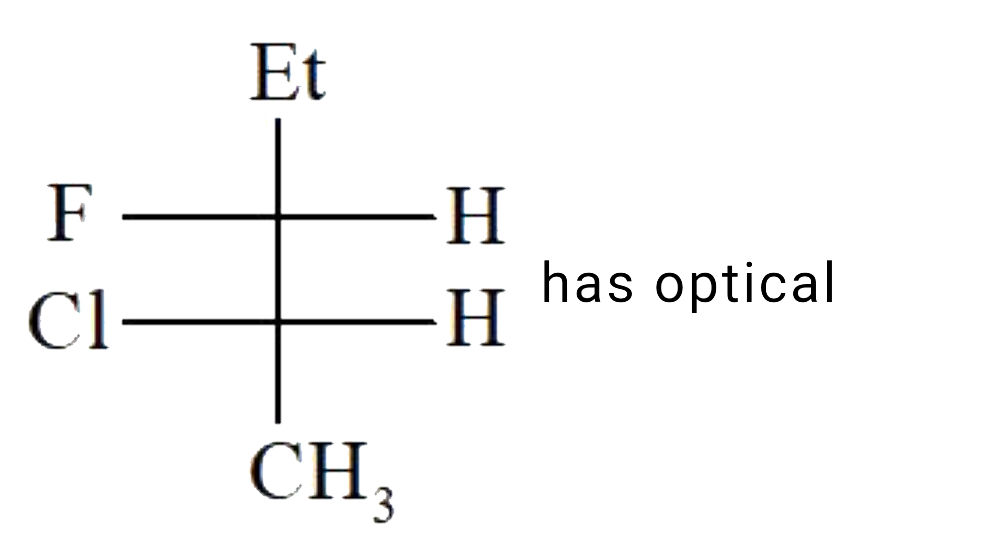
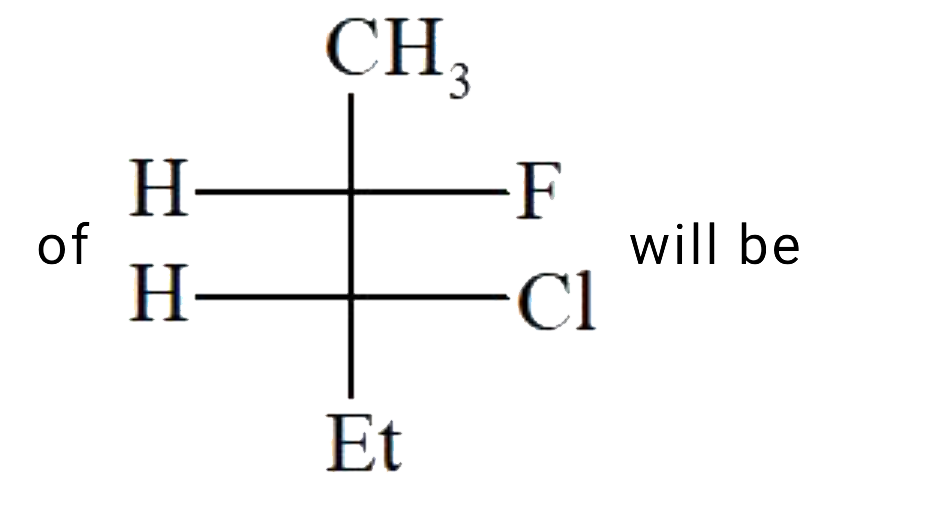
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- rotation -45^(@), so optical rotation of
Text Solution
|
- Specific Rotation And Conditions For Optical Activity
Text Solution
|
- If optical rotation produced by is +36^(@), then that produced by
Text Solution
|
- The change of optical rotation of glucose solution with time is refere...
Text Solution
|
- Which of the following have the same value of optical rotation ?
Text Solution
|
- If optical rotation produced by is 36^(@) then that produced by is :
Text Solution
|
- rotation -45^(@), so optical rotation of
Text Solution
|
- प्रकाशीय सक्रिय यौगिकों का प्रकाशीय घूर्णन होता है
Text Solution
|
- Killer Revision Of Rotation In 45 Minutes
Text Solution
|