A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
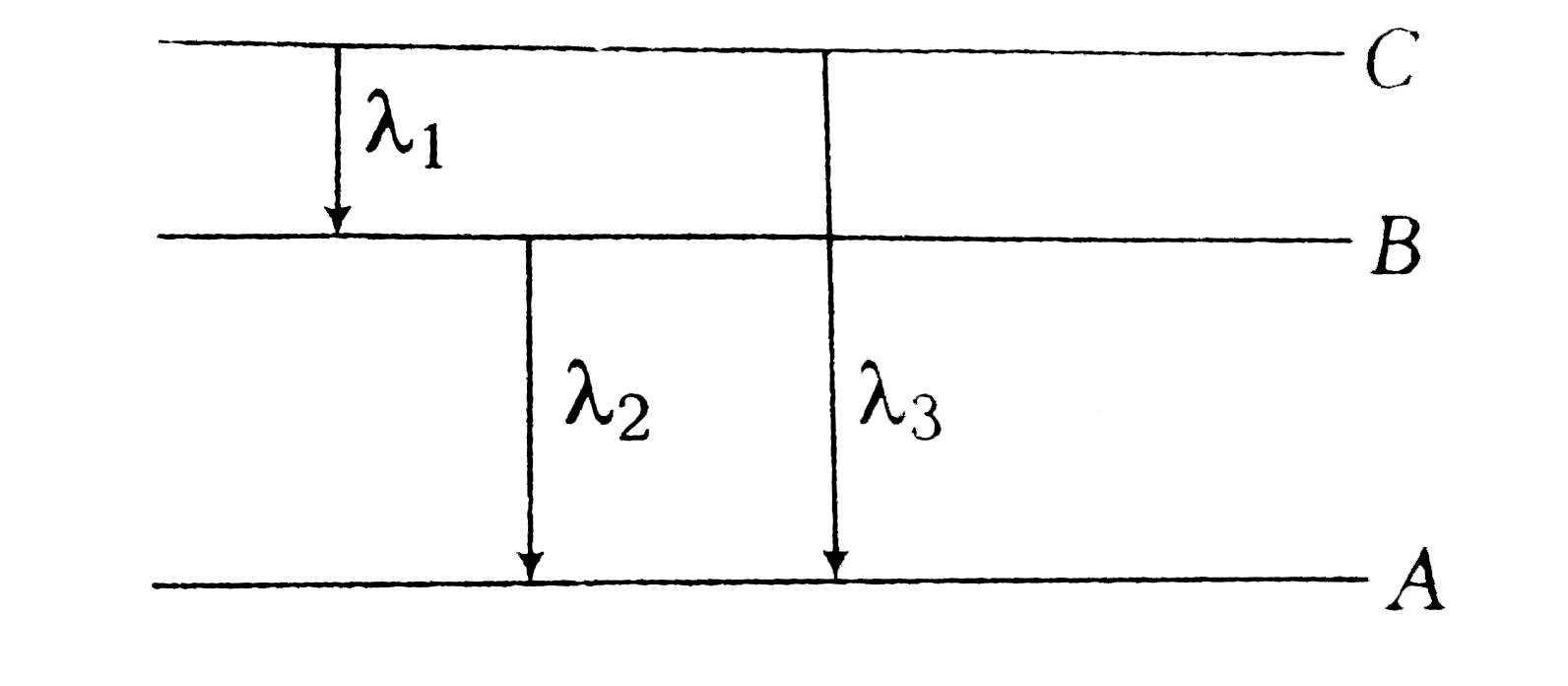
- Energy levels A, B and C of a certain atom corresponding to increases...
Text Solution
|
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- The energies of energy levels A, B and C for a given atom are in the s...
Text Solution
|
- Three energy levels P,Q,R of a certain atom are such that E(P)ltE(Q)lt...
Text Solution
|
- For a certain atom, there are energy levels A,B,C corresponds to energ...
Text Solution
|
- Energy levels A,B,C of a certain atoms corresponding to increasing val...
Text Solution
|
- Energy levels A, B and C of a certain atoms correspond to increasing ...
Text Solution
|
- Energy levels A,B,C of a certain atom correspond to increasing values ...
Text Solution
|
- किसी परमाणु के ऊर्जा स्तर A, B व C की ऊर्जाएं क्रमशः E(A),E(B),E(C) है...
Text Solution
|