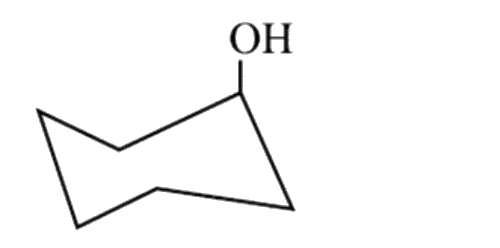
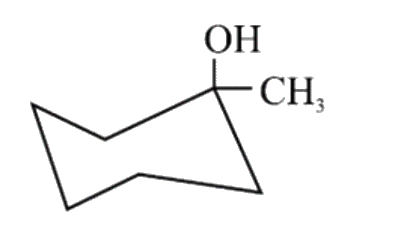
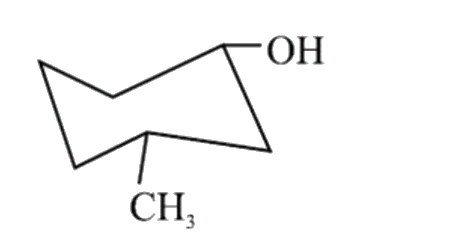
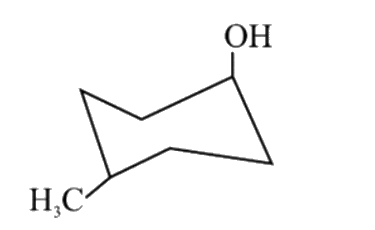
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following react with HBr at faster rate ?
Text Solution
|
- Which of the following will react faster with Lucas reagent ?
Text Solution
|
- HBr reacts faster with:
Text Solution
|
- Which of the following reacts fastest with HBr?
Text Solution
|
- Which of the following reacts fastest with HBr?
Text Solution
|
- Amongest the following, HBr reacts fastest with
Text Solution
|
- Which will react faster with NBS?
Text Solution
|
- Amongst the following , HBr reacts fastest with .
Text Solution
|
- HBr निम्नलिखित में से किस के साथ सबसे तीव्र अभिक्रिया करता है?
Text Solution
|