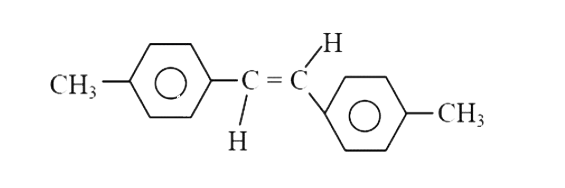
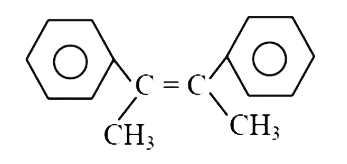
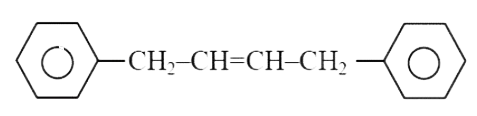
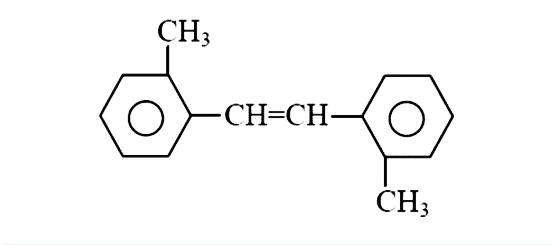
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An alkene (A)C(16)H(16) on ozonolysis gives only one product (B)(C(8)H...
Text Solution
|
- An alkene (A) C(16)H(16) on ozonolysis gives only one product (B) (C(8...
Text Solution
|
- An alkane (A) C(16)H(16) on ozonolysisi gives only one products (B) C(...
Text Solution
|
- (a) Give the general formula of an : (i) alkane,(ii) alkene,(iii) alky...
Text Solution
|
- An alkene (A) C(16)H(16) on ozonolysis gives only one product (B) C(8)...
Text Solution
|
- An optically active compound having molecular formula C(8)H(16) on ozo...
Text Solution
|
- Compound C(4)H(8)CI(2) (A) on hydrolysis gives a compound C(4)H(8)O(B)...
Text Solution
|
- An alkene (A) C(16)H(16) on ozonolysis gives only one product (B) C8H8...
Text Solution
|
- An organic compound (A) of molecular formula C(5)H(8) when treated wit...
Text Solution
|